FDA’s patient labeling specific resources on this webpage are primarily directed to industry staff who develop patient labeling for prescription drugs. For assistance on how to navigate this webpage and the associated FDA labeling resource webpages for human prescription drugs see video. For more information about:
FDA-approved patient labeling includes:
Not all prescription drugs are required to have FDA-approved patient labeling.
In May 2023, FDA published a proposed rule about a new type of FDA-approved patient labeling, known as Patient Medication Information (PMI).
Consumer medication information (CMI) is written information for patients and caregivers about a prescription drug that is developed by an individual(s) or organization other than the drug company. FDA does not review or approve CMI, and the drug company does not review CMI.
A Medication Guide is patient labeling that is part of the FDA-approved prescription drug labeling for certain prescription drugs when the FDA determines that:
Medication Guides (MGs) are developed by applicants, approved by FDA, and required to be distributed to patients. The medication guide shall be dispensed to the patient (or to the patient’s agent) in paper form when the product is dispensed; however, the patient may also request electronic delivery of the MG in lieu of the printed form.
MG resources include:
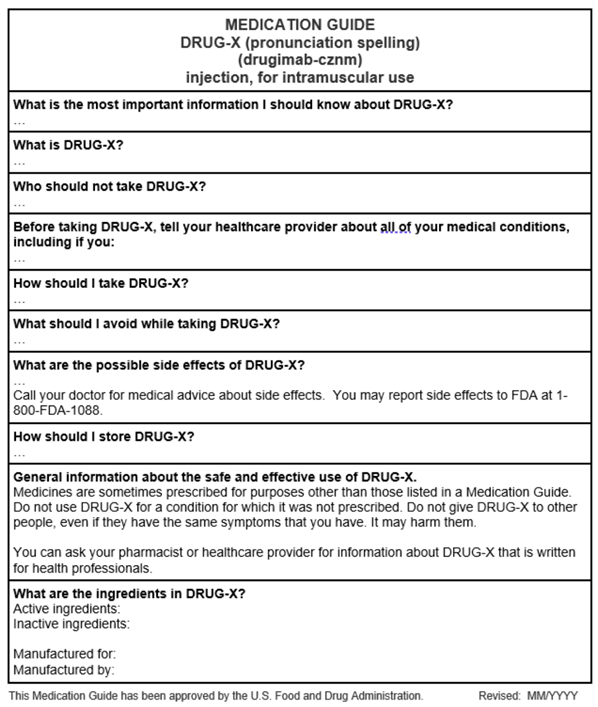
Below see an example MG template that provides a general overview of the MG (this MG does not contain any product-specific information). Consider using the enclosed Sample Template for Medication Guides when developing a Medication Guide (this sample template may not contain all the Medication Guide requirements or guidance recommendations).

A Patient Package Insert (PPI), also known as “Patient Information” is patient labeling that can be part of the FDA-approved prescription drug labeling. Certain PPIs are developed by the manufacturer and approved by the FDA.
PPIs are required for oral contraceptives and estrogen-containing products, and these PPIs are required to be dispensed with each prescription:
PPIs for other prescription drugs are submitted to the FDA voluntarily by the manufacturer and approved by the FDA, but their distribution is not mandated.
A guidance that has specific recommendations for the PPI is the Child-Resistant Packaging Statements in Drug Product Labeling (final guidance).
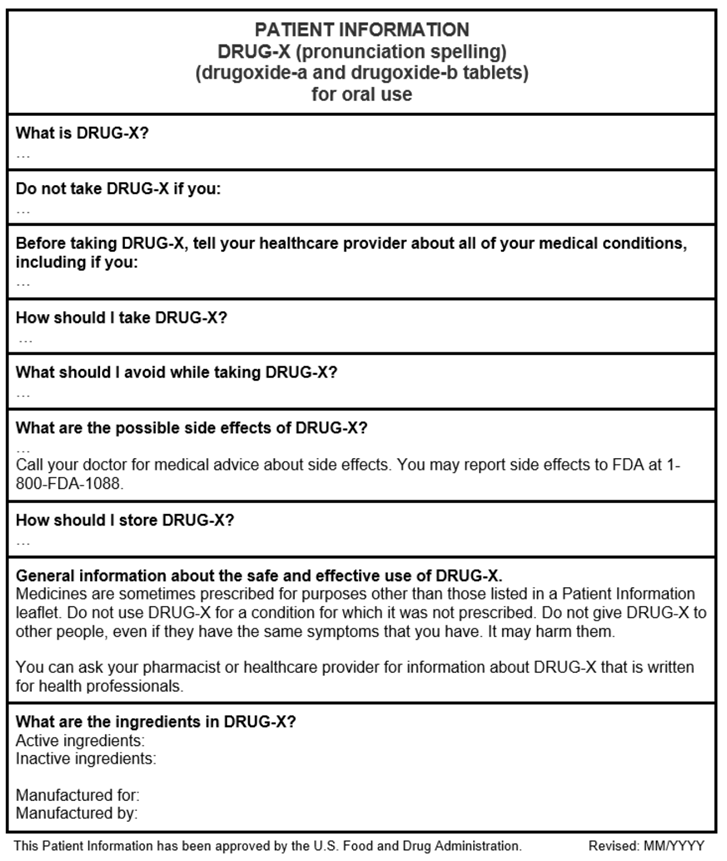
Below see an example PPI template that provides a general overview of the PPI (this PPI does not contain any product-specific information). The headings in this example PPI may not be applicable to all PPIs. Consider using the enclosed Sample Template for Patient Package Inserts when developing a PPI (this sample template may not contain all the PPI requirements or guidance recommendations).
The Instructions for Use (IFU) is patient labeling that can be part of FDA-approved prescription drug labeling for a biologics license application (BLA), a new drug application (NDA), or an abbreviated new drug application (ANDA). The IFU is developed by applicants for patients (or their caregivers) who use prescription drugs that have complicated or detailed patient-use instructions. The IFU provides detailed, action-oriented, step-by-step written and visual instructions for the patient on how to use the drug including instructions on preparation, administration, handling, storage, and disposal. The IFU is developed by the applicant, reviewed and approved by FDA, and provided to patients (or their caregivers) when the drug is dispensed. Some IFUs are not approved by the FDA. IFU resources include:
Below see an example IFU template that provides a general overview of the IFU (this IFU does not contain any product-specific information).

FDA published a proposed rule, Medication Guides: Patient Medication Information in May 2023. Under the proposed rule, FDA is proposing to amend its human prescription drug labeling regulations to require a new type of Medication Guide — Patient Medication Information (PMI) — for prescription drugs used, dispensed, or administered on an outpatient basis, as well as for blood and blood components transfused in an outpatient setting. When the rule is finalized, PMI will:
For additional information on the proposed PMI rule visit the Patient Medication Information webpage.
For specific application or supplement questions or for general questions about prescription drug labeling, please visit Prescription Drug Labeling Contact Information.
For general questions about patient labeling or this website please email CDEROMP@fda.hhs.gov.